At the 2025 ASCO congress it was presented the positive results of the PANOVA-3 trial. This is the first and the only positive trial in locally advanced pancreatic cancer (LA-PDAC)
Tumor treating fields (TTFields) use alternating electric fields to disrupt cancer cell proliferation.
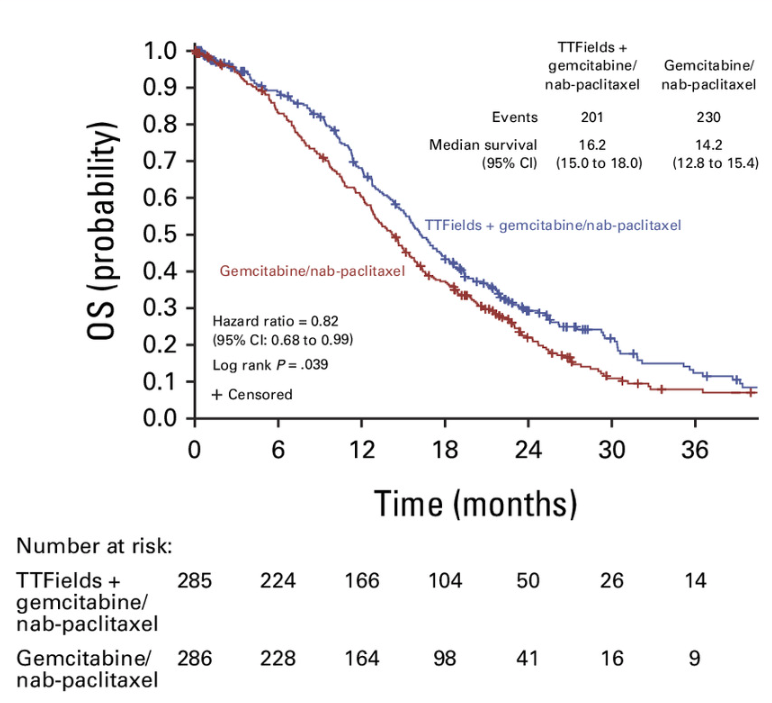
571 newly diagnosed LA-PDAC were randomly assigned to received gemcitabine and nab-paclitaxel with or without TTFields.
Overall survival was significantly prolonged using TTFields with chemotherapy versus chemotherapy alone (median 16.2months, vs 14.2 months, HR 0.82, p 0.039).
Progression free survival, local progression free survival, and overall response rate were not improved.
Importantly pain free survival was significantly prolonged with TTFields plus chemotherapy (median 15.2 months vs 9.1 months, HR0.74, p 0.027).
Distant progression free survival was longer in patients treated with TTFields plus chemotherapy (13.9 months vs 11.5 months, HR 0.74, p 0.022).
Device-related skin adverse events were experienced by 76.3% of patients, but only 7.7% of patients presented grade 3 adverse events.
This trial was published in J Clin Oncol on May 31, 2025, and the lead author of the study is Dr. Teresa Macarulla, a distinguished member of ALIPANC.
The full study can be accessed at the following link: https://pubmed.ncbi.nlm.nih.gov/40448572/